Why Is Water Less Dense as a Solid
Waters lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes. Solid water or ice is less dense than liquid water.

Water Less Dense As Ice Why Lakes Don T Freeze Solid Winter Math Winter Math Kindergarten Ice Video
This means ice floats on water.

. The water molecules are pushed farther apart compared to liquid water. Waters lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes. That the solid phase is less dense than the liquid phase is an.
Solid water ice is less dense than its liquid state. The water molecules are pushed farther apart compared to liquid water. Solid water or ice is less dense than liquid water.
Water is one of the few substances on Earth that is less dense as a solid than a liquid. I want to make sure that I understand why ice is less dense than water in its liquid state so please correct me if I am wrong. In this state the water molecules are actually further apart than when they are in a liquid state.
This is essential for aquatic life. The reason is hydrogen bonds. Begingroup Are you asking about specifically elemental substances all the examples you give except water are elements.
Ice is less dense as a solid than as a liquid ice floats Liquid water has hydrogen bonds that are constantly being broken and reformed. Why is solid water less dense than liquid water. Since ice is less dense than liquid water it floats to the top of of the water.
Ice is less dense than. Alcohol is less dense than water so spirits can float on top of water or juices. In water the hydrogen bonds have the ability to.
Density is the mass per unit volume of a material. The liquid phase is less dense than the solid phase but more dense than the gas phase and the gas phase is less dense than either the solid or liquid phases. Water is more dense than alcohol or oil because its.
The density of water is roughly 1 gram per milliliter but this changes with temperature or if there. Water is unusual in that its maximum density occurs as a liquid rather than as a solid. Liquid water is less dense than ice because water molecules get bonded to each other in the solid state through hydrogen bonds which are very directional that means they show specific.
What happens as water freezes. The density of ice is 09168gcm-3 at 0 C The density of liquid water is 09999gcm-3 at 0 C. In practical terms density is the weight of a substance for a specific volume.
Why is water less dense as a solid than a liquid. When were talking about water when were talking about water when we go to the liquid state when we go from liquid water to solid ice to solid ice we actually get we actually get less. Ice is less dense than.
This is why water expands as it freezes and is less dense than the surrounding liquid water. As ice water molecules form four hydrogen bonds that lock them into a rigid crystalline structure. Ice is less dense than water because the hydrogen bonds orientation causes the molecules to push farther apart from each other.
When water freezes water molecules form a crystalline structure maintained by hydrogen bonding. Water is an exception. Or any possible compound in which case giving a list might be a.
Why is water more dense than alcohol. When water is frozen into ice the change in temperature creates excess hydrogen bonds between water molecules that increase the space between the molecules. As water cools so the hydrogen bonds align to cause the water molecules to become aligned and each molecule takes up more space so the solid is less.
Why is Water Less Dense as a Solid. When water freezes water molecules form a crystalline structure maintained by hydrogen bonding. Each molecule forms stable hydrogen bonds and creates a 3-D crystal.

Concept Cartoon This Concept Cartoon Titled Added Energy Could Be Used To Assess Students On Their Understanding Of Particle Understanding Particles Energy
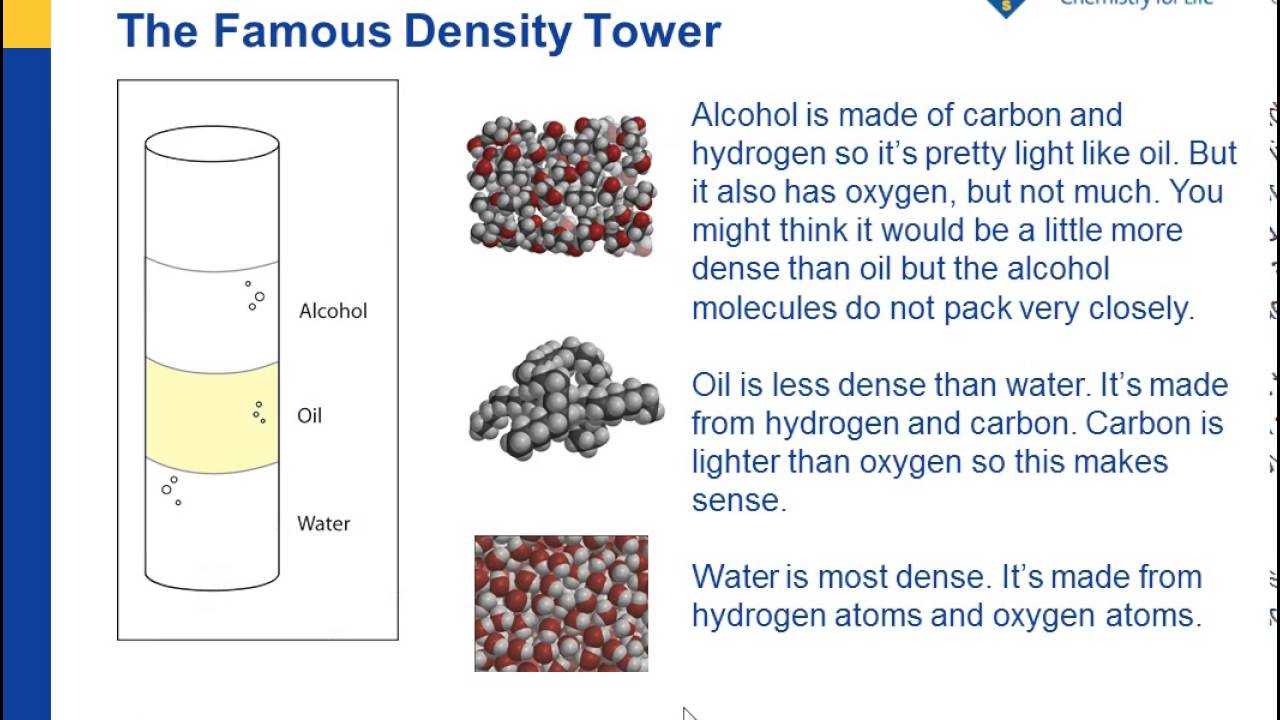
Density Sink And Float For Liquids Chapter 3 Density Middle School Chemistry Middle School Chemistry Teaching Chemistry Chemistry

Because Of Hydrogen Bonding Water Is Less Dense As A Solid Than It Is As A Liquid Consequently Ice Floats Chemistry Outline Biology

Ice Floats Because Solid Water Is Less Dense Than Liquid Water Arctic Landscape Landscape Outdoor
Comments
Post a Comment